13C NMR Examples
The 13C chemical shift is dependent both on the presence of electronegative groups and on the steric environment. This is best demonstrated by examining a variety of hexane isomers:
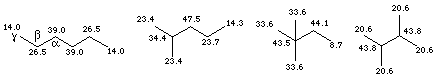
Simple interior (primary and secondary) carbons tend to be in the range d 25 - 45. Methyl groups which terminate unbranched alkyl chains, however, are significantly shielded (moved to lower d values), as shown by the examples above (d 14, 14.3 and 8.7). The origin of this effect is thought to be steric compression in the gamma (g) position due to gauche interactions. This is shown schematically below and the gamma position is marked above in the example for hexane.
The presence of an electronegative atom such as oxygen tends to move the chemical shift of the a-carbon down into the region d 65 - 90, as shown in the examples below:
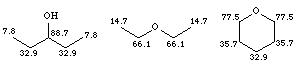
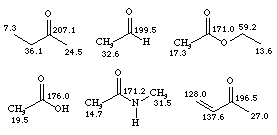
Halogens, however, have effects which are difficult to predict and carbons adjacent to halogens tend to have chemical shifts in the d 30 - 50 region, as shown below. The effects are not simply additive, however, and multiple substitution can often be shielding (move the signal to lower d values). The nitrile carbon is significantly shielding and adjacent carbons tend to occur in the d 20 - 25 region.
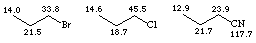
Alkene carbons tend to have chemical shifts in the range d 110 - 140, as shown in the examples below. Conjugation between alkene centers has little effect, as demonstrated by the two middle structures shown below. Conjugation with an oxygen, however, has a dramatic shielding effect, which is attributed to contributions from the resonance forms shown below.

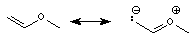
Alkyne carbons occur in the region d 65 -85, and are significantly shielding to the carbons which are immediately adjacent (d 1.5 for the terminal methyl of 2-pentyne).
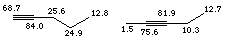
Carbonyls are the most highly deshielded carbons which are typically encountered. Their intensity is usually weak, since there are no attached hydrogens to contribute to the Nuclear Overhauser Effect enhancement (with the exception of aldehydes). Typical chemical shifts occur in the region d 170 - 210 with esters, carboxylic acids and amides at the low end, and simple ketones and aldehydes at the high end of the range.
Aromatic carbons have chemical shifts in the range d 120 - 140 and are shifted within this range by the nature of the attached substituent. The multiplicity of aromatic peaks in the non-decoupled spectrum is useful for identifying aromatic substitution patterns.
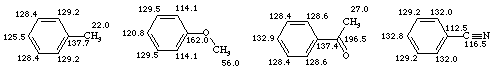
Copyright 1998, Brooks/Cole Publishing Company