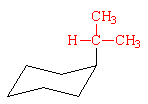
Note how the H of the isopropyl group is pointed back over the ring rather than a methyl group. This minimizes the steric strain from 1,3-diaxial interactions and is the reason why the isopropyl group (axial strain energy = 2.2 kcal/mol) is not much larger than a methyl group (axial strain energy = 1.7 kcal/mol).