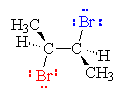
Reactants
A Br2 molecule (red and blue) is preparing to add to an molecule of cis-2-butene. The Br2 approaches from below (or above) the plane of the atoms of the double bond because this is the location of the electron density of the pi bond. As the red Br leaves, the blue Br becomes electrophilic and adds to the pi bond. The pi electrons of the double bond form a bond from one C to the electrophilic Br and a pair of electrons on the Br forms a bond to the other C of the double bond, forming the bromonium ion. This step of the reaction results in a syn addition.

Bromonium Ion Intermediate
The bromoniun ion has a three-membered ring of two carbons and a bromine, with a positive charge on the Br. Because both bonds to the blue Br are formed from the same side of the plane of the double bond, the methyl groups, which were cis on the double bond of the reactant alkene, are still cis on the ring of the product (syn addition). The reaction of the nucleophilic Br- to open the ring is an SN2 reaction and occurs with inversion of configuration at the C-Br bond that is broken.
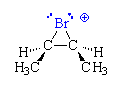
Products
Because the bond between the C and the blue Br in the bromonium ion is broken by the nucleophilic Br- (green) in an SN2 reaction with inversion of configuration, the product has the two bromines on opposite sides of the plane of the original double bond. Thus, the addition occurs with anti stereochemistry. This product is (2R,3R)-2,3-dibromobutane, and results from the nucleophilic Br- attacking one of the two carbons (the C on the right corner of the structure as shown on page 417) of the bromonium ion. Of course, the nucleophilic Br- can also attack the other carbon of the bromonium ion. This gives the product shown below.
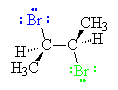
This time, the nucleophilic Br- is shown as red. The product, which results from attack of the nucleophilic Br- at the left corner of the ring, is (2S,3S)-2,3-dibromobutane. It is the enantiomer of the product shown above. The nucleophilic Br- cannot tell any difference between the two carbons of the bromonium ion, so reaction at either is equally probable. The reaction produces a 50:50 mixture of the two enantiomers. The product is racemic, as it must be since the starting materials are not chiral. Note, however, that the reaction has not produced any of the diastereomeric meso-isomer. The reaction is stereospecific. A similar reaction of (E)-2-butene produces only meso-2,3-dibromobutane. It is also stereospecific.